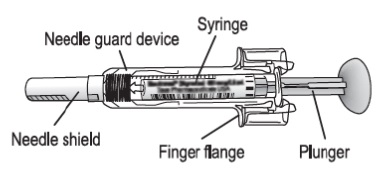
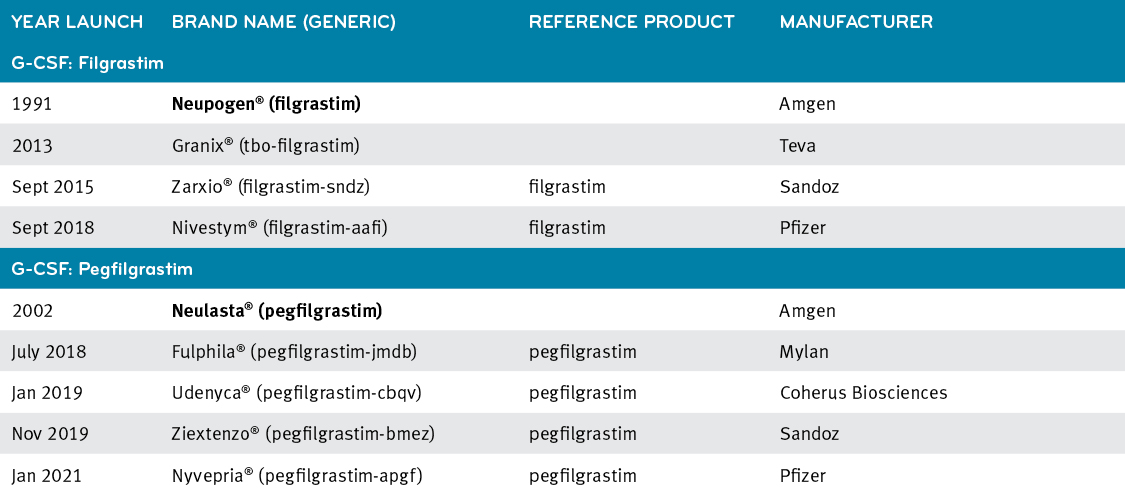
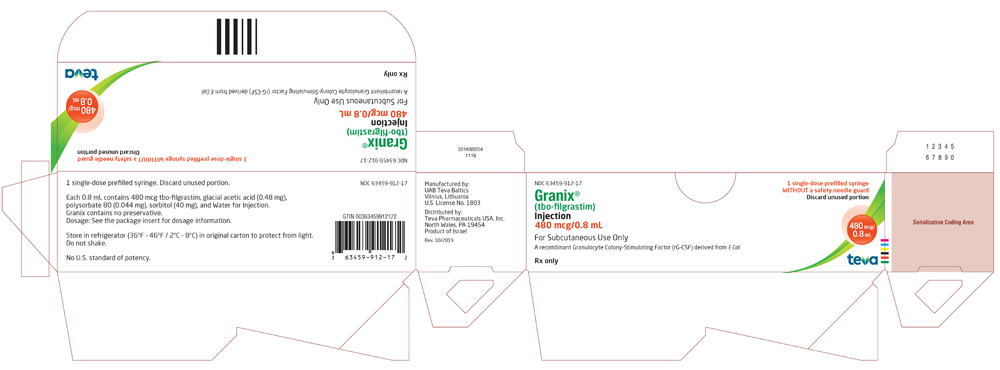
Real-world safety experience of tevagrastim/ratiograstim/biograstim and tbo- filgrastim, short-acting recombinant human granulocyte colony-stimulating factors | Request PDF
Final Appraisal Report Filgrastim (TevaGrastim®▽) Teva UK Limited Advice No: 1410 – August 2010 Recommendation of AWMSG